18.Argus 50 Ca2+ imaging manual
M.Aihara 19960210
This equipment is a Ca2+ imaging analyzer. You can have an intracelllular Ca2+ dinamics as a two dimensional color image.
Argus 50 system
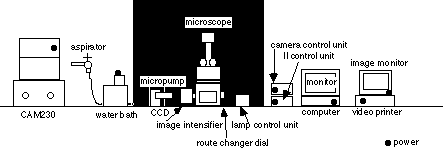
equipment
1. CAM230 UV lamp and UV filter (filter 1 & 2)
exite fluorescence
2. aspirater with water pump, aspirate solution from chamber
3. micropump irrigate ligand solution or wash
4. water bath keep desireble temperature of assay buffer
5. lamp control unit for bright field microscope, variable type
6. microscope Nikon TMD300
7. camera control unit control contrast or sensitbity
8. image intensifier integrate fluorescent image
9. II control unit control sensitibity
10. computer and monitor Argus 50 control unit
11. image monitor show image
12. video printer output of monitor image
preparation of sample
To get Ca2+ image, cells are needed to culture on glass coverslips with flexiperm disc. Coverslips are needed to be washed, autoclaved and coated by desireble materials. In details, please refer 'Protocol of hippocampal neuronal culture'.
Fura2-AM loading
Fura 2 loading is most important step of this Ca2+ imaging. Loading time and Fura2 concentration are necessary to be examined every for cells. Essential loading procedures are following.
required materials:
Fura2 -AM(Dojindo) Wako 343-05401(DMSO 1mM)
stock 20μl each in -20℃ (lab No5 corridor)
Hepes-tyrode BSA 0.1%
conc. MW g/l
NaCl 140mM 58.4 8.2
KCl 2.7mM 74.5 0.2
CaCl2 1.8mM 147 0.26
NaHCO3 12mM 84 1.0
D-glucose 5.6mM 180 1.0
NaH2PO4 0.37mM 156 0.058
Hepes 25mM 238 6.0
for neuronal culture (Mg free!!)
MgCl2 0.49mM 203 0.1
pH7.4 (NaOH) and filter through
BSA 0.1% (Pentex)
loading time: Neuron 30min-60min
astroglia 2-3hours
microglia 30min
CHO 1hour-*
Cos7 3hour-*
NRK 30min
*: these cell lines are difficult to be loaded by Fura2 AM
note: After loading in 37℃ incubator, methyl-esteration of acetoxymethyl in cells may proceed to be left in room temperature for about 1 hour, especially in CHO or Cos7 cells.
loading procedure:
1. take small amount of Fura2AM with pasteur pipettes, attach a few microlitter of it on the wall of 15ml-cetrifuge tube with loading buffer(HEPES-tyrode BSA0.1%) and vortex well, and repeat this step
note:if Fura2 loading buffer isn't mixed well, Fura2 grains are left at the bottom
2. replace medium to loading buffer not to dry the surface of cell culture
3. leave culture in CO2 incubator at 37 ℃
Ca2+ imaging
1. power on (figure sign ●)
2. control on the monitor
C:\>
input 'ca' and return
then Argus 50/CA start up
select 'CA(Fura2)'
3. select 'camera-CA2' by pulling down 'image' on menu bar
4. select imaging parameters
4-0 stop sequence input how many images you get in this assay
4-1image store
select 'disk' if you monitor ratio image in your experiment, you must select 'disk'. Fast sampling is available if you select 'flame memory' but you cannot monitor ratio image.
4-2sampling intervals
'normal' single pattern of sampling interval, set your desirable interval time
'double' variable patterns of sampling intervals, I usually set 10 and 30 secs interval times.
4-3real time ratio monitor
You can monitor real time ratio image of your experiment on this mode. If you want, please select 'imaging' and click 'setup'
'ratio range' min 0.5 max 5.0 is normal parameter, If cells can respond well to Ca2+, max ratio over 5.0 is better.
'real time ratio imaging' select 'color image display' , it is better not to select 'save ratio image' because this mode waste time and memory.
5.set your coverslip on the table of microscope
5-1 If you need irrigation and aspiration of the well, you must set both tube in the well. Then check micropump and aspirator.
The tip of aspiration needle should be 2 or3 mm above the bottom, if not , it's possible that the cell surface dry up by aspiration.
The tip of irrigation needle should be below the surface of medium and above the bottom because a drop from the tip to the surface or strong flow disturb image or damage cells.
![]()
6.check following steps to get UV light and fluorecent image
6-1 UV lamp on
6-2 objetive lens for fluorescence
6-3 filter for Ca2+ imaging (filter box is under objective lens revolver)
7.select your desirable field by microscope (route change dial is "A")
8.select 'OK' on the monitor and change route changer dial of microscope from "A" to "C"!
9.Fluorescent image is on the monitor. control sensitivity to get the favorable intensity of fluorescence. dial up the sensitivity until slight red dot appear in the image. Loading is better, sensitivity is lower. Best loading sensitivity is below "5.0".
10.click 'OK' then select 'color' in the monitor window
11.set threshold to cut the background . If loading is better, better contrast image is available. Favorable threshold level is below 30.
12.click 'OK' then 'dark image aquisition' window is open. select 'OK'
13.click 'start' and once more
14.real time ratio image should appear every preset interval
15.If you change interval, click 'change'. So next interval will be changed.
16.If you end your assay, click 'stop'.
17.If you mark stimulation time, click 'mark'. Next marking number is indicated in this window.
18.save image with file name
To make ratio image file and its analysis, please consult original manual or M.Aihara!!
note: ligand stimulation is very important step in this assay
*don't open the black curtain when you inject ligand solution
*don't touch the well or coverslip because image slip out of the frame
*apply slowly not to make strong flow to the cell surface because some kind of cells can respond
*diffusion of ligand solution is slow! 3 or 4 folds volume of ligand solution for the liquid volume in the chamber should be applied.



